Definition of Flow Cytometry
"Cyto"
- Greek for hollow/cell (‘kytos’)
- Greek for hollow/cell (‘kytos’)
"Metry"
- Greek for the action or process of measuring ("metria")
“Cytometry is a process for measuring the physical and chemical characteristics of biological cells. In flow cytometry the measurements are taken as cells flow through the instrument in a fluid stream.”
Howard Shapiro, Practical Flow Cytometry 4th ed.
A quick note about FACS
F - Fluorescence
A - Activated
C - Cell
S - Sorting
A - Activated
C - Cell
S - Sorting

- More properly known as as flow cytometry
- FACS is a brand name and trademark of BectonDickinson
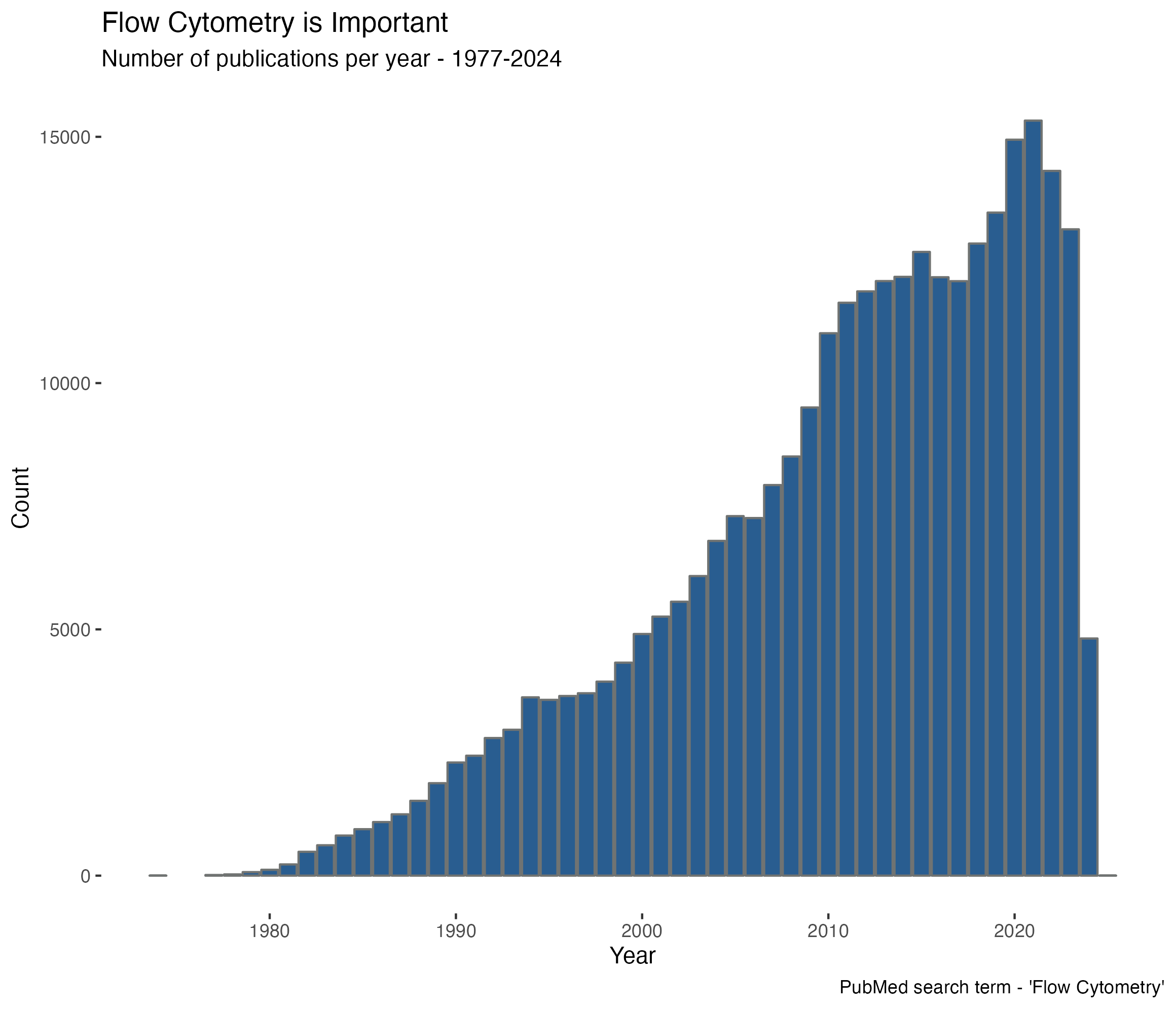
Flow Cytometry is core to numerous studies
- Number of publications on PubMed mentioning "flow cytometry "
Sorters vs. Analysers
Analysers acquire information from cells

Sorters can separate specific populations of cells

Cytometry
versus
Microscopy
Microscopy - Overview
Flow Cytometry - Overview
- Light
- Slide
- Fluorescence
- Filters
- Observation
- Laser
- Fluidics
- Fluorescence
- Filters
- Electronics
- Quantitation
Flow Cytometry - 2 paradigms for detection
Conventional Flow Cytometry
- One channel = One Fluorophore
- ~18-28 fluorescent parameters
Spectral Flow Cytometry
- Many Detectors = Many Fluorophores
- 15-40 parameters (and theoretically much more)
Flow Cytometry - A detector for every "colour"
For each fluorophore we want to visualize we need a separate Photo Multiplier Tube (PMT), filter set and channel to record the data
Conventional Flow Cytometry partitions the spectrum
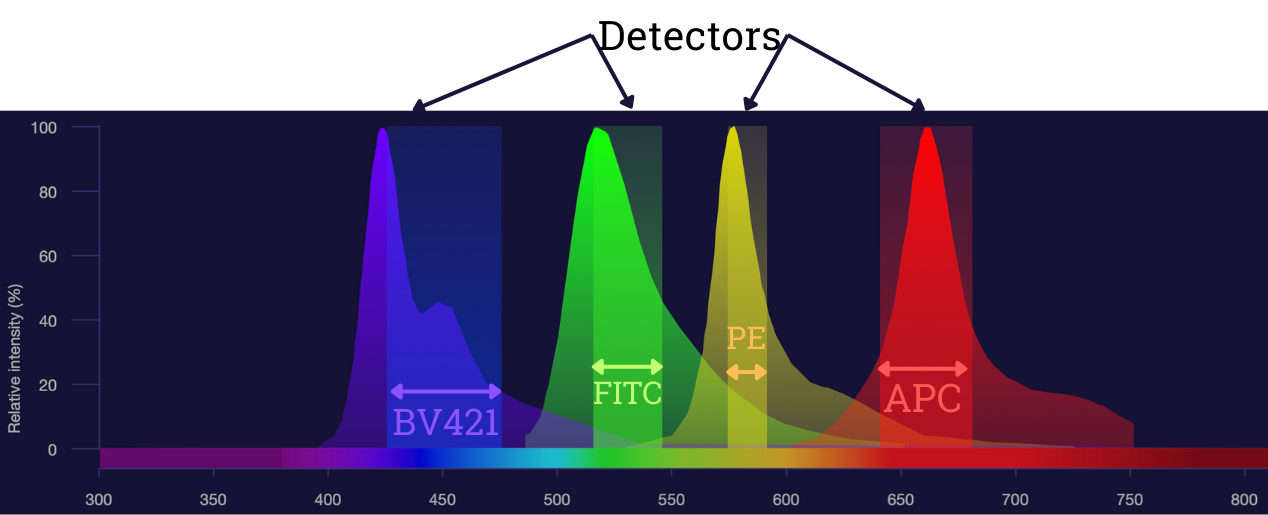
Chop up the spectrum into sections and associate the light from each section with a specific fluorophore
Spectral Flow Cytometry looks at everything
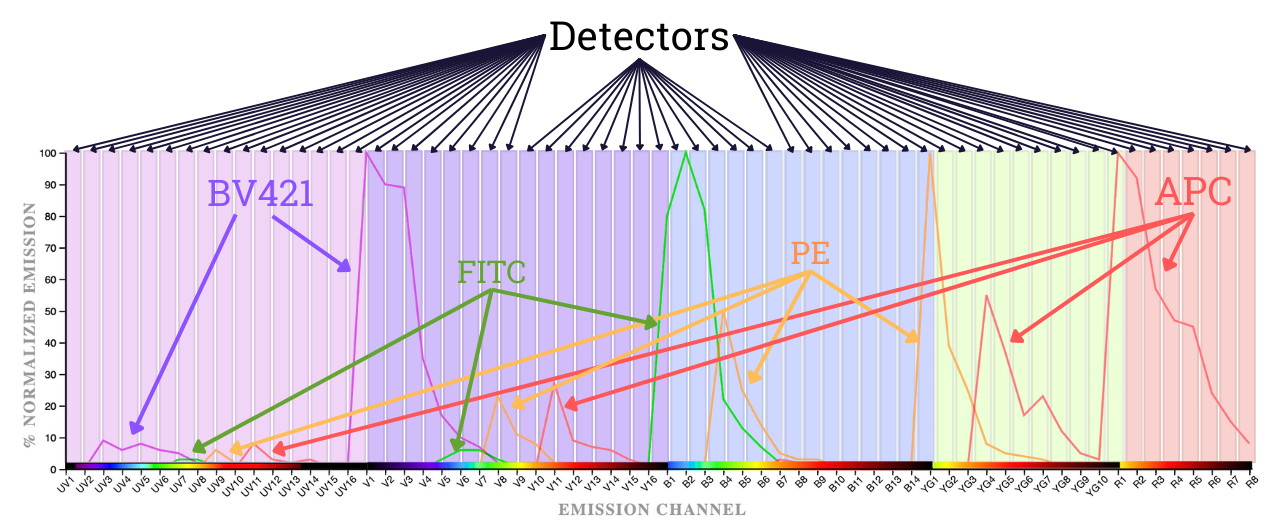
Look at all of the spectrum all of the time. Identify a fluorophore from the unique fingerprint it has across the entire visual spectrum
What does flow cytometry data look like?
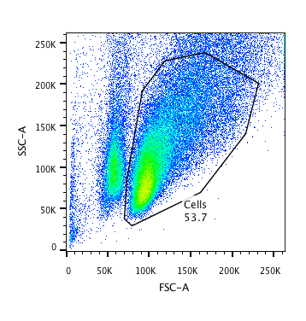
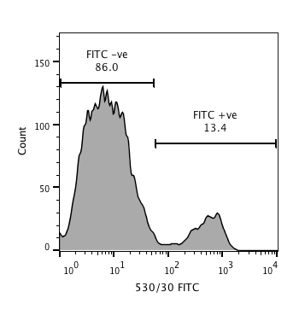
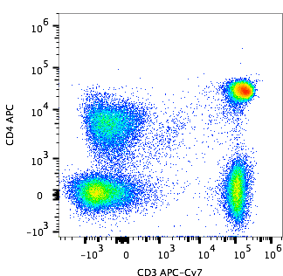
What are the Benefits of Flow Cytometry?
- Thousands of events analyzed in a short period of time
- Statistical information obtained very quickly
- Flexibility of data acquisition
- Ability to re-analyze
Main uses include:
- Phenotyping
- Pharmacokinetic and Pharmacodynamic assays
- DNA Analysis
- Functional Studies
- Fluorescent Proteins
- Cell Sorting
Flow Cytometry is all about fluorescence
If it's fluorescent we can see it

If we can see it we can sort it!

Fluorescence
Fluorescence
Intrinsic Fluorescence
- Autofluorescence
Extrinsic Fluorescence - Fluorescence YOU add
- Antibodies
- Dyes
- Fluorescent Proteins
The Electromagnetic Spectrum
1
2
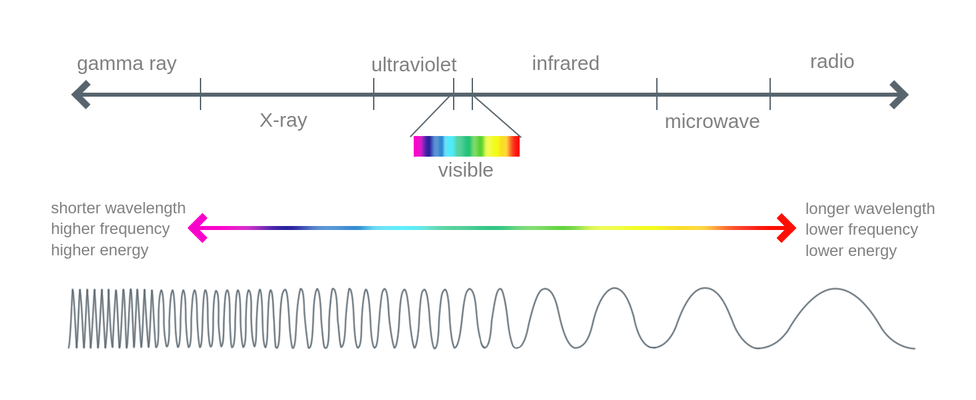
Laser Wavelengths
Fluorescence - Physical Properties
Fluorochromes by Application
Fluorochromes for Antibody Labelling
- FITC
- Phycoerithrin (PE)
- Allophycocyanin (APC) PE-Cy5, PE-Cy7
- AlexaFLuors
- Brilliant dyes (Violet, UV, etc.)
Functional Probes
- Indo-1
- Fluo-3
- Thiazole Orange
- Pyronin Y
- SNARF
- TMRE
Fluorochromes by Application
Viability and DNA Markers
- Propidium Iodide
- DAPI
- Hoechst dyes
- Acridine Orange
- DyeCycle dyes
- TOPRO-3
- DRAQ dyes
- Fixable Live-Dead dyes
Fluorescent Proteins
- Cyan FP
- Green FP
- Yellow FP
- Red FP
Fluorescent Spectrum
- Absorption vs Emission
- Excited by one wavelength (dotted line)
- Emits at another (higher) wavelength (solid line)
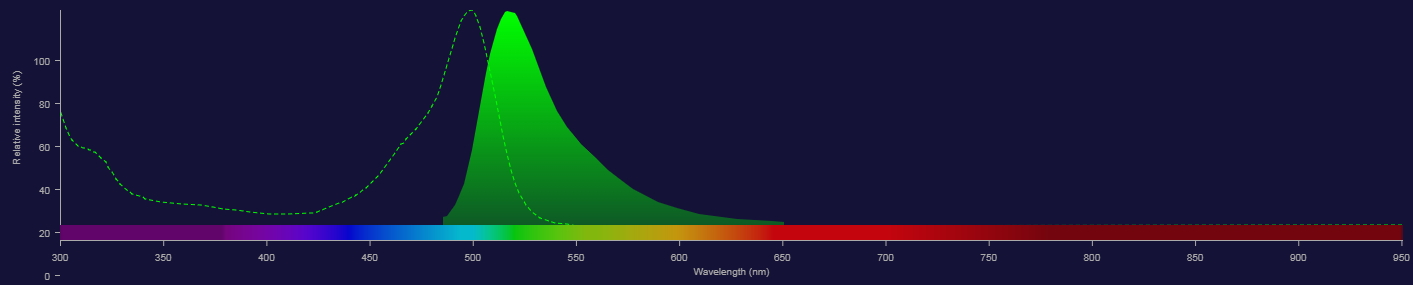
Fluorescence (capturing emission)
With one fluorochrome this is easy
- Collect all fluorescent light above the absorption spectrum knowing it is derived from our fluorophore
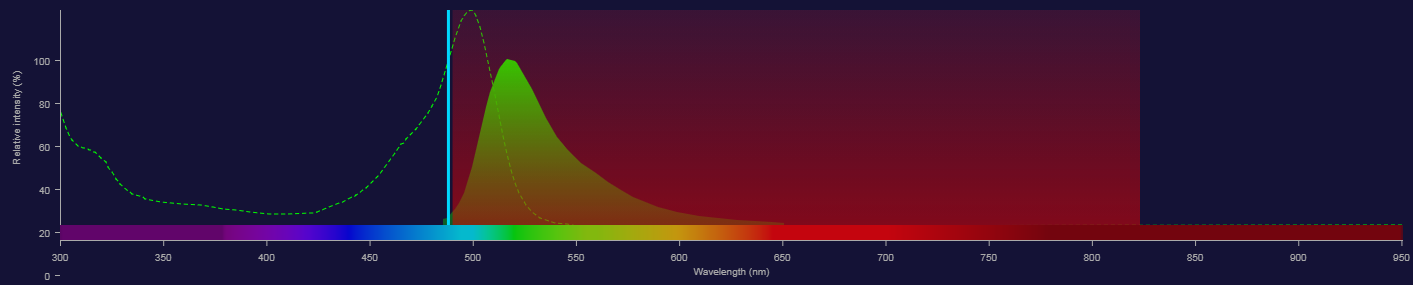
Fluorescence - Adding another fluorochrome
- How about PE?
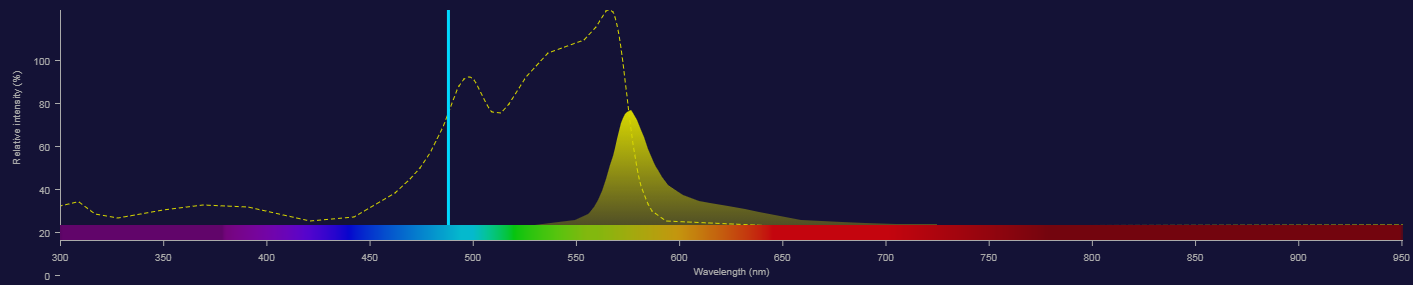
Fluorescence - Adding another fluorochrome
With 2 fluorophores we can't collect all of the fluorescence
- How would we tell which photons came from each fluorophore?
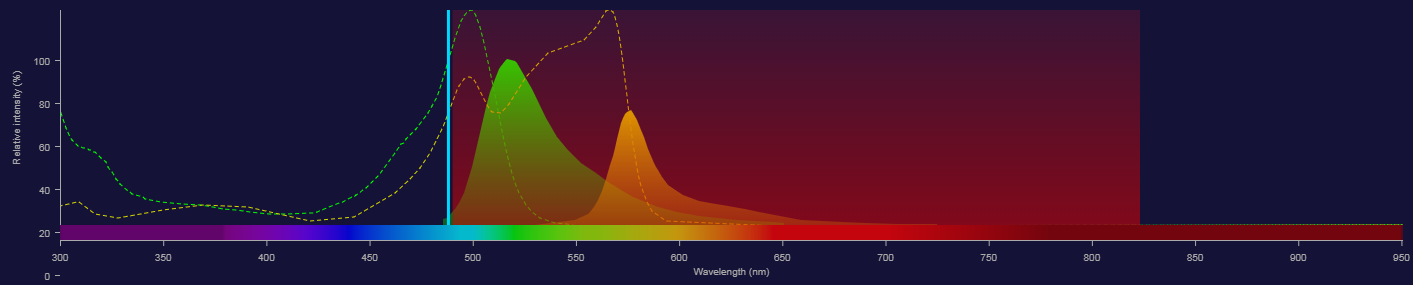
Fluorescence - Adding another fluorochrome
- We can filter the light with optical filters.
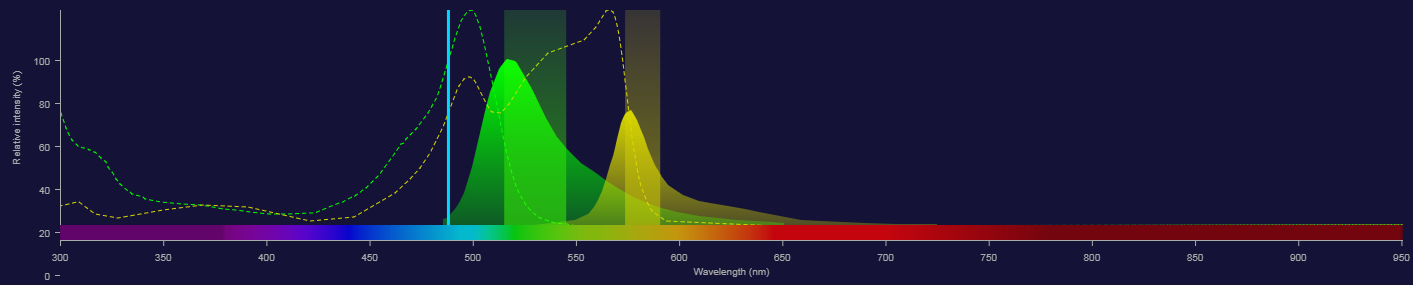
Fluorescence: Adding Another Fluorochrome
- Even if we are very selective with filters there will always be some light collected from the other/another fluorophore.
Fluorescence: Adding Another Fluorochrome
- Spectral overlap like this can be dealt with by controls and compensation
- Practical training will cover this
Types of Optical Filters
Filters transit light of specific wavelengths, whilst reflecting others.
There are 3 main types of optical filters
- Longpass - LP
- Shortpass - SP
- Bandpass - BP
Types of Optical Filter
500SP (Short Pass)
500/50 BP (Band Pass)
500LP (Long Pass)
Stacking filters
- By combining LP and BP filters we can:
- Broadly Fractionate the spectrum reaching a PMT with Long Pass or Short Pass Filters
- Precisely filter the light in a narrow band with a Band Pass filter
- This allows us to use multiple PMTs on each laser line
- Broadly Fractionate the spectrum reaching a PMT with Long Pass or Short Pass Filters
- Precisely filter the light in a narrow band with a Band Pass filter
Stacking Filters
- All light collected from the event is directed to the detectors
- BP/LP stack extracts green light for measurement by PMT
- BP/LP stack extracts light blue light for PMT assessment
- Violet light is diverted for downstream assessment
Fluorescence - Summary
To design ANY fluorescence experiment we need to know:
- Excitation Spectrum
- Will determine whether we can use the fluorophore
- Do we have lasers suitable to excite the fluorophore
- Emission Spectrum
- will tell us which filter to use and whether we can combine the fluorochrome with others.
Fluorochromes
Fluorochromes for Antibody Labelling
Fluorochrome Structure
- Cyclic ring compounds
- FITC, Texas Red, Alexa dyes,
Propidium iodide, Hoechst - Tandem dyes
- PE-Cy5, APC-Cy7
- Fluorescent proteins
- GFP, YFP, CFP etc
- Nanocrystal dyes
- Q Dots
- Polymer dyes
- Brilliant Violet, Brilliant UV
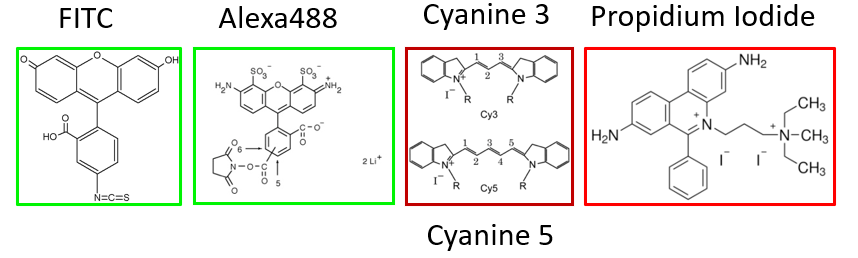
Fluorochromes - Cyclic Ring Compounds
- FITC, Texas Red, Alexa 488, Propidium Iodide, Hoechst
- Generally small molecules
Fluorochromes - Tandem Dyes
- FRET (Fluorescence Resonance Energy Transfer)
- A combination of a donor and acceptor molecule
- Emission of donor and excitation of acceptor must overlap
- Donor transfers energy (not fluorescence) to acceptor
- Effective between 10-100Å only
Fluorochromes - Tandem Dyes
- Tandem dyes were developed to expand the range of colours when cytometers were less sophisticated.
- Use DONOR and ACCEPTOR molecules
Fluorochromes - Tandem Dyes
Will show fluorescence from the donor in addition to the acceptor
- This can get "worse" over time
- Due to oxidation of the tandem
- PE-Cy5
- PE-Cy5.5
- PerCP-Cy5.5
- APC-Cy5.5
- PE-Cy7
- PerCP-Cy7
- APC-Cy7
Fluorochromes - Tandem Dyes
Storage is critical
- Effect of light - Highly photosensitive
- Effect of fixatives
Lot-to-lot variation is a significant concern.
- Each lot will have different spectral properties requiring instrument settings to be adjusted.
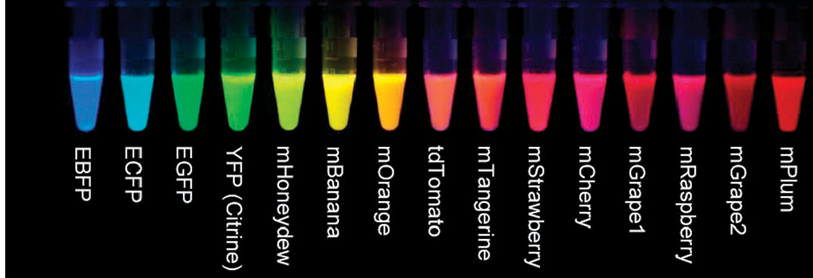
Fluorochromes - Fluorescent Proteins
Derived in the early 90's
- GFP
- RFP
- CFP
- BFP
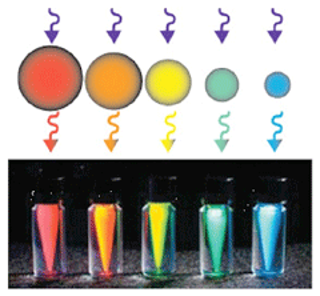
Fluorochromes - Nanocrystals
- Q Dots (Quantum Dots) – mid to late 1990s
- Size of crystal determines wavelength emitted
- Larger the crystal the redder the light
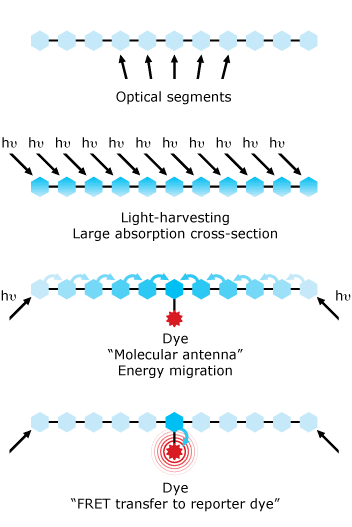
Fluorochromes - Polymer Dyes
- New class of dyes - early 2000s
- Use ‘molecular antennae’
- High Quantum efficiency
- Bright
- Brilliant Violet
- Brilliant Ultraviolet
- Brilliant Blue
- Brilliant YG
- Brilliant Violet
- Brilliant Ultraviolet
- Brilliant Blue
- Brilliant YG
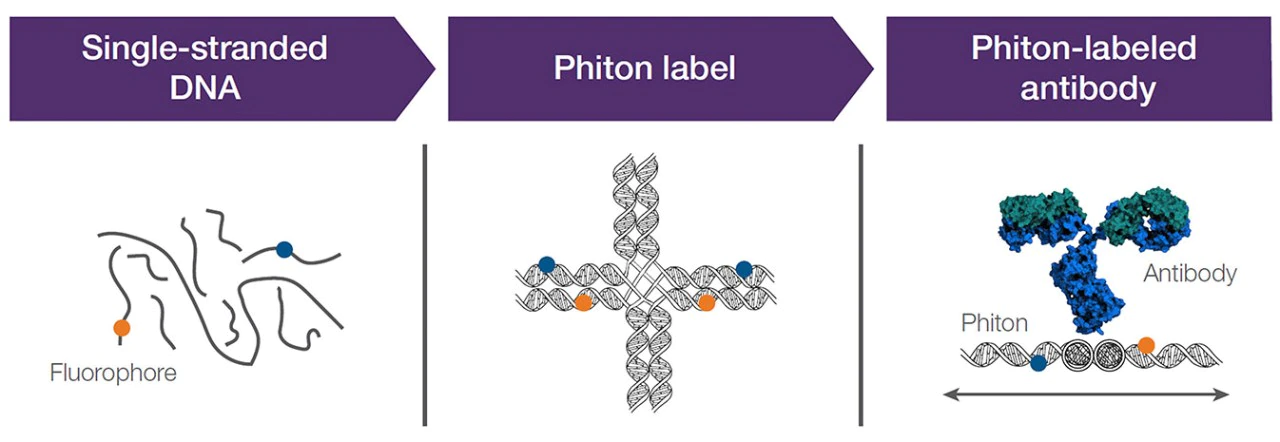
Fluorochromes - Nova Fluors - the other polymer dyes
- New spin on polymer dyes - ~2017
- Single stranded DNA backbone
- less cross-laser spillover
- 45+ panel fluorescence flow cytometry demonstrated
- DNA binding dyes interfere
- Fixable L/D only
- DNA binding dyes interfere
- Fixable L/D only
.webp)
Fluorochromes - Properties
To assess a fluorochromes potential in flow cytometry we have to assess its "brightness ", which is dependent on:
- Excitation characteristics
- Emission characteristics
- Extinction coefficient – how strongly it absorbs photons
- Quantum yield – how many photons on average come out
Fluorochromes - Properties
So how do we assess fluorochrome brightness?
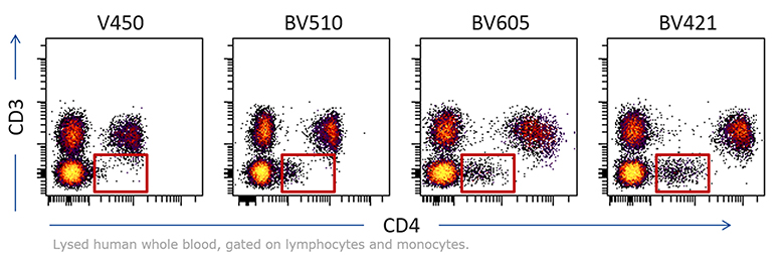
- Measure how far positive cells are from negatives (different fluorophores)
- Stain Index
- Separation Index
Stain Index = (MFIpos- MFIneg) / (2*SD)
Fluorochrome Brightness
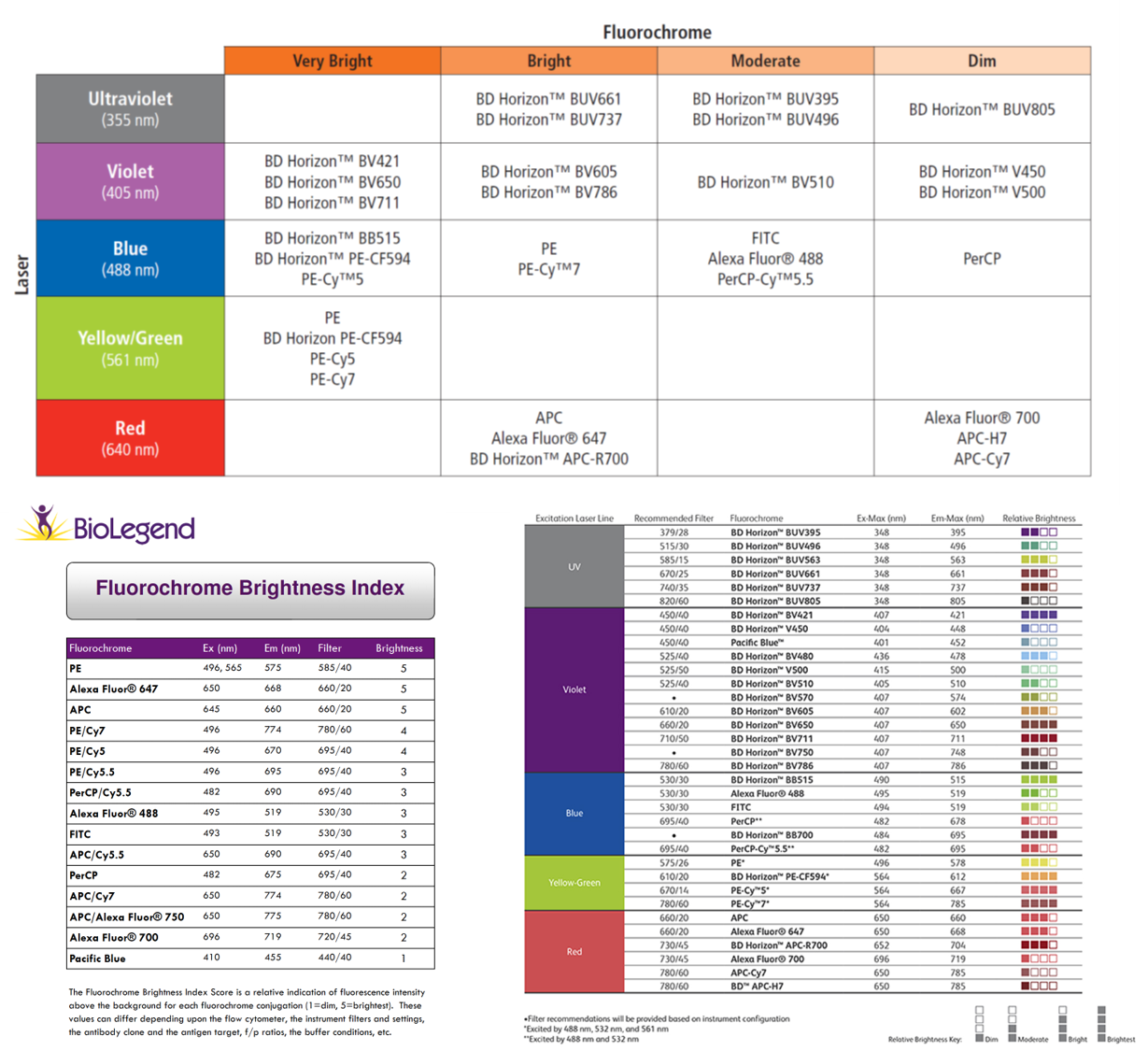
Fluorochrome brightness can be looked up on BD, Biolegend, Thermo etc sites if you don’t want to measure.
Fluorochrome - Classes
Why do I need to know this?
- The choice will influence which application you can use
- Some fluorochromes require special handling
- To multiplex, the dye chemistry will be important
- Think about Fixation
- Location of antigen
- Closeness of antigens
How a cytometer works
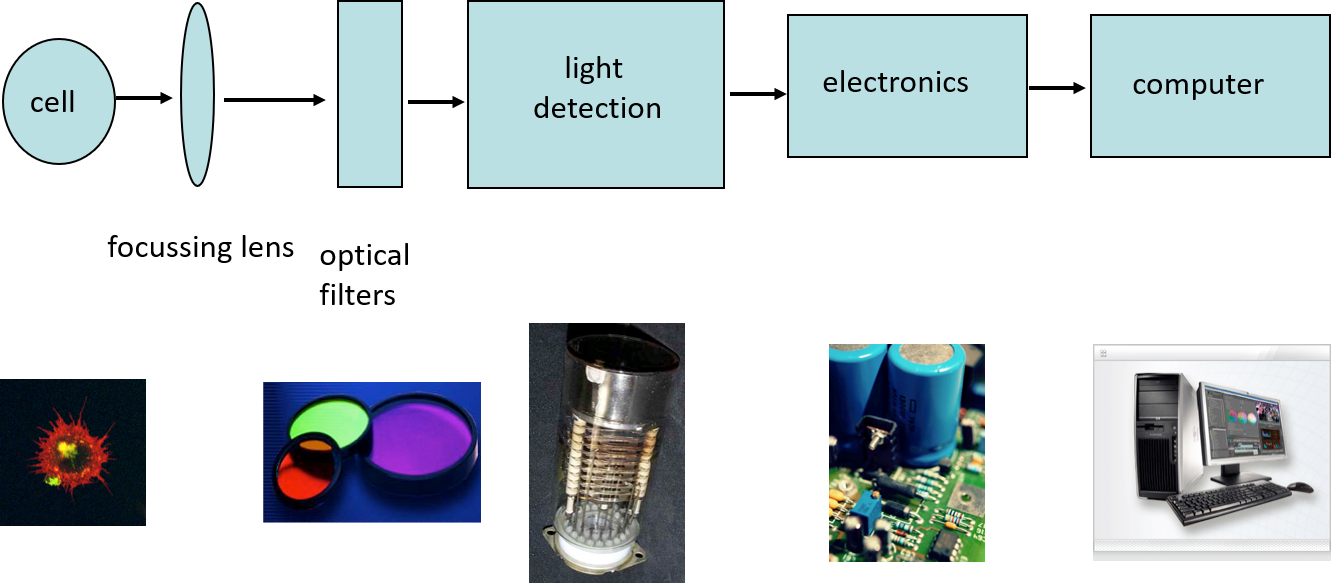
How does a cytometer work?
- Laser
- Fluidics
- Optics
- Filters
- Electronics
- Quantitation
Cytometer Components
- Fluidics:
- Separation and alignment of particles, sorting, analysis and sorting speed.
- Optics:
- Light source(s), detectors, spectral separation (filters, dichroic mirrors).
- Detectors:
- Collection and analysis of optical signals; data display; light source(s), detectors and sorting setup and control.
Cytometer Components
- Data Analysis:
- Data display and analysis;
- Multivariate analyses;
- Identification of subpopulations;
- Quantification
Cytometers: Differential Pressure Systems
- Differential Pressure machines e.g. LSRII, Fortessa
- Both the sample and sheath fluids are pressurised
- Flow rate is set by sheath pressure and is controlled by the difference between sheath and sample pressures
- Absolute Counts not possible without external addition e.g. Fluorescent Counting Beads
Cytometer - Sheath Fluid
- Sheath fluid (usually FACSFlow) is used to deliver the cells from a tube to the flow cell of the machine.
- It also aligns the cells to allow them to be individually analysed in the centre of the laser beam in the flow cell

Cytometer - Laminar Flow
- Viscous drag at walls slows outer layers of liquid
- Particles are drawn toward centre of channel – hydrodynamic focusing
- The sheath fluid surrounds the sample in concentric layers.
- These layers do not mix.
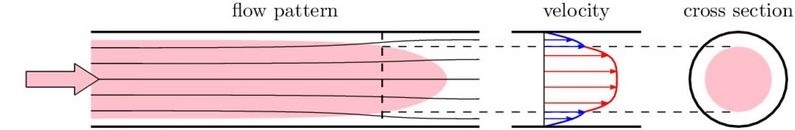
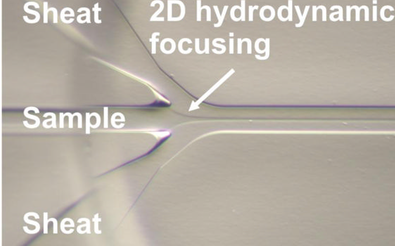
Cytometer - Hydrodynamic Focusing
- Aligns the cells so they go
- one by one
- through the centre of the laser beam (where the intensity is greatest)
- It pulls cells out like a ‘string of pearls’
- one by one
- through the centre of the laser beam (where the intensity is greatest)
What Happens when you press 'Hi'
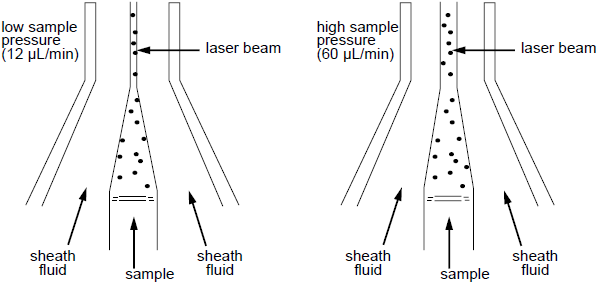
What Happens when you press 'Hi'
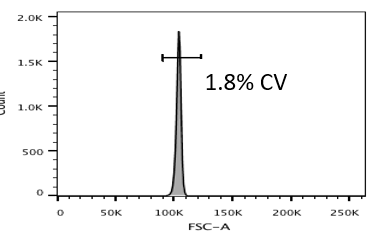
Low
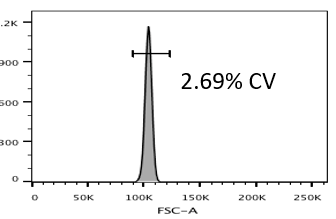
High
Cells enter the lasers Path
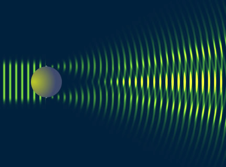
Light Amplification by Stimulated Emission of Radiation
- Wavelengths from UV (325nm) to Infrared (780nm).
- Most cytometers will have more than one.
- Fortessa has 5
- Properties of laser light
- Coherent= narrow spectrum ie only emit a single colour of light
- Divergent = beam doesn’t increase in width away from source
- Polarised = Light has the same handedness
- Lasers may be co-linear (measured simultaneously) or separated (measured temporally and spatially separately).
- Coherent= narrow spectrum ie only emit a single colour of light
- Divergent = beam doesn’t increase in width away from source
- Polarised = Light has the same handedness
- Lasers may be co-linear (measured simultaneously) or separated (measured temporally and spatially separately).
Cytometer - What do we detect?
- Scattered laser light
- Fluorescent emission

Cytometer - what do we measure
Cytometer - what do we measure
Cytometer - Scattered Light
- Signal influenced by
- Cell size
- Refractive Index
- Nuclear : Cytoplasmic ratio
- Granularity
- Surface topography
- Correlates only
- Cell size
- Refractive Index
- Nuclear : Cytoplasmic ratio
- Granularity
- Surface topography
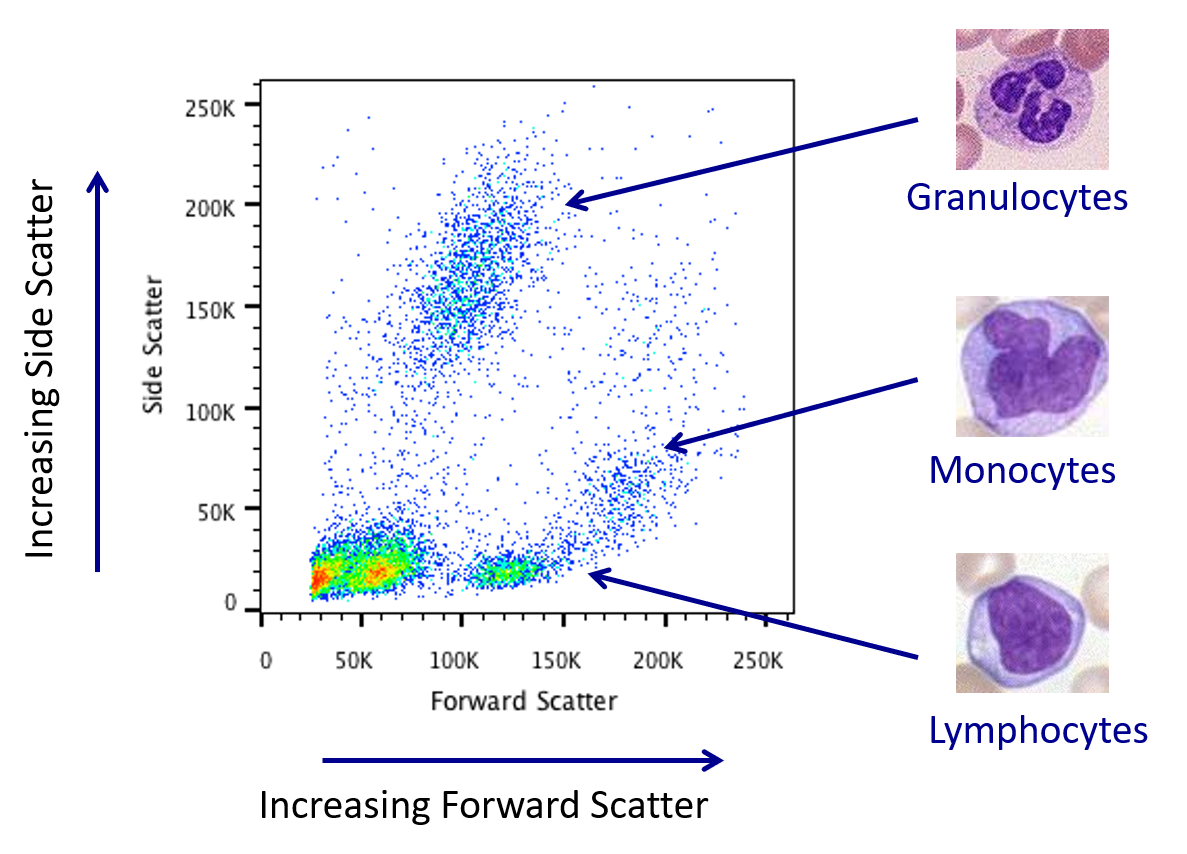
Cytometer - Scattered light
- What does whole blood look like?
- A heterogeneous population
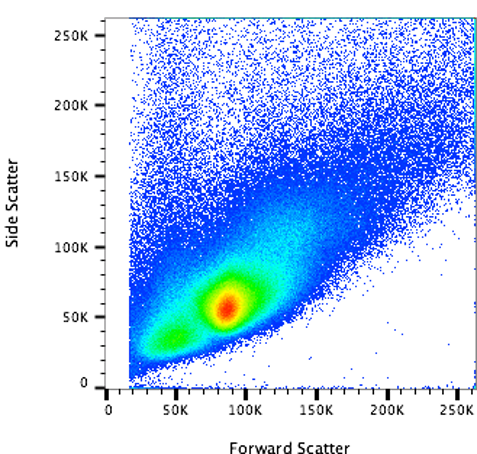
Cytometer - Scattered Light
- What does a cell culture look like?
- More homogeneous
Cytometer - Fluorescence Emission Detection
- Fluorescence is emitted in all directions but is collected at 90°
- Optical elements (filters and dichroic mirrors) separate wavelengths and direct them to different pathways
Cytometer - Fluorescence detection
- Remember the spectral viewer
- How does that translate into how the instrument is set up?
- How does that translate into how the instrument is set up?
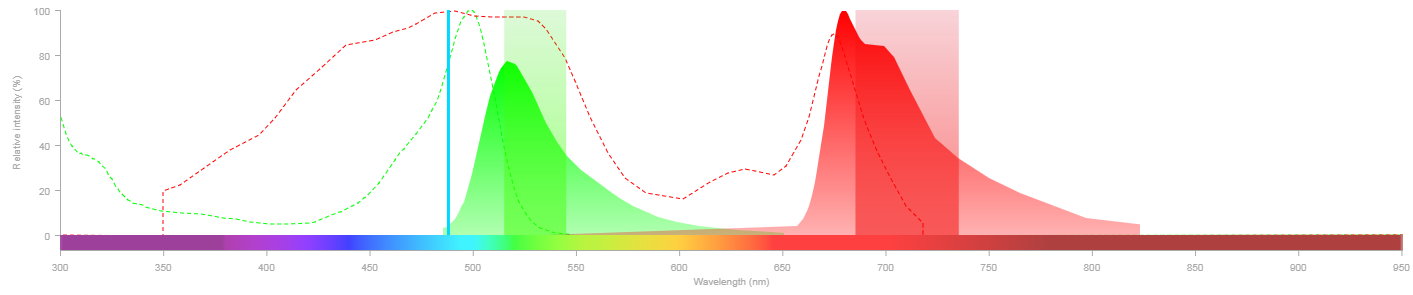
Cytometer - Fluorescence detection
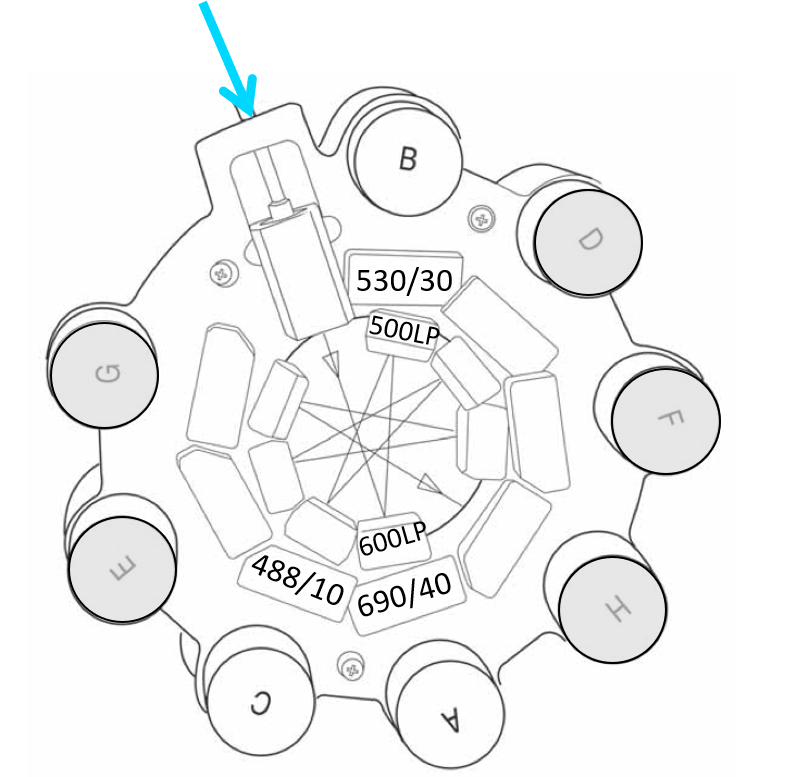
- Red light collected first
- lowest energy
- lowest energy
Cytometer - Detectors
- Photodiode
- Used for bright signal, when saturation of detector is a potential problems e.g. FSC
- Photomultiplier tubes (PMT)
- More sensitive than a photodiode e.g. Fluorescence detector
- PMT/PD convert light energy into an electrical signal
- Used for bright signal, when saturation of detector is a potential problems e.g. FSC
- More sensitive than a photodiode e.g. Fluorescence detector
Cytometer - Detectors - PMT
- PMTs are ‘blind’ – they simply detect photons
- We have to optically filter the light
- Photon energy is converted to a signal that is dependent on:
- Number of photons hitting the PMT
- The voltage we apply to the PMT
- The measurement is only relative
Set up of PMT voltage is extremely important
Controls are extremely important
- We have to optically filter the light
- Photon energy is converted to a signal that is dependent on:
- Number of photons hitting the PMT
- The voltage we apply to the PMT
- The measurement is only relative
Set up of PMT voltage is extremely important
Controls are extremely important
Cytometer - Electronics System
Cytometer - How histograms and dotplots are made
- listmode table of data
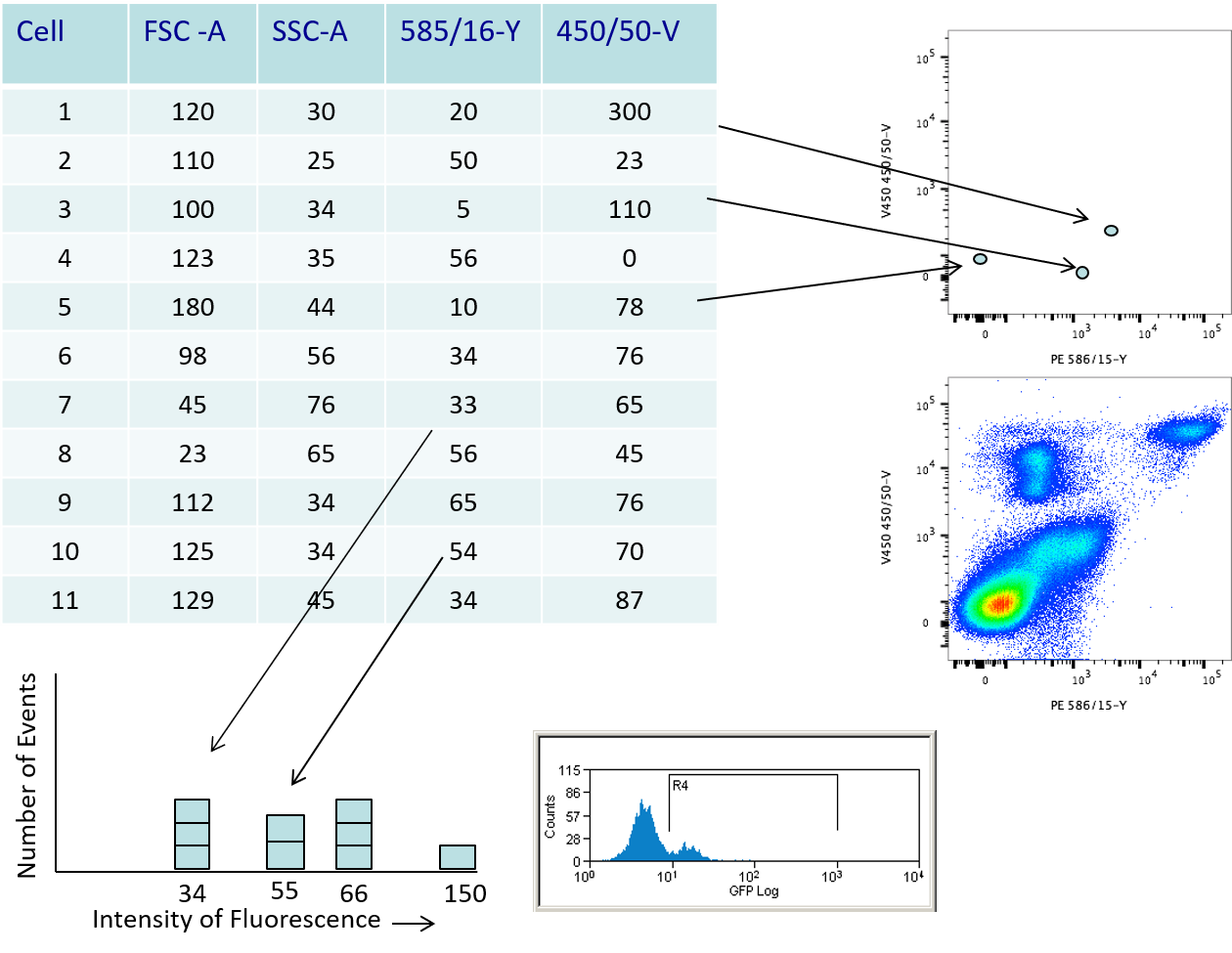
- Plotted for every cell
- Builds up density
- The histograms and dotplots we refer to are really density plots
Cytometer - Why do I need to know this?
- I won’t be designing a flow cytometer anytime soon!
- But YOU WILL need to know how the flow cytometer you use is designed:
- To understand how to design experiments
- To determine which instruments that are available to you will work with the fluorochromes you need to use for specific applications
- To understand how to design experiments
- To determine which instruments that are available to you will work with the fluorochromes you need to use for specific applications
Cytometer - Summary
Sample Preparation
Sample Preparation
- Sample preparation is the single most important factor in running a good experiment
- Single Cell suspension is imperative
- Cells need to be viable (unless fixed)
- Should be little debris
- Fluorescence detected should be specific
- Your experiment is only as good as your sample preparation
- Single Cell suspension is imperative
- Cells need to be viable (unless fixed)
- Should be little debris
- Fluorescence detected should be specific
- Your experiment is only as good as your sample preparation
Dissociating Cells
- Suspension cells e.g. Jurkat cells
- Cell lines, blood, bone marrow
- Can use Ficoll or similar gradient media
- If sticky, EDTA or DNAse in medium may help
- If using blood, consider lyse red cells
- Ammonium chloride or Commercial reagents
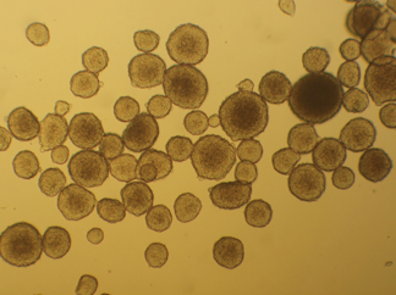
Dissociating Cells
- Adherent cells
- Cell lines
- Detachment using trypsin, collagenase, EDTA, Accutase
- Be aware of viability
- DNAse or EDTA in medium
- Differential centrifugation
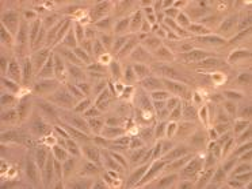
Dissociating Cells
- Solid Tissue
- Biopsy, paraffin section
- Digestion or mechanical disaggregation
- Collagenase, trypsin, pronase
- May depend on antigen
- Cells v nuclei
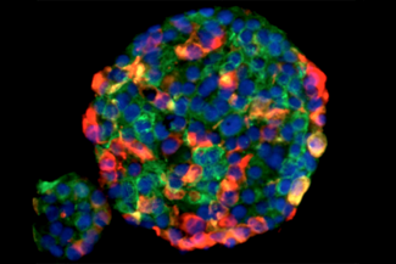
Sample Preparation
- Balance between dissociation and viability
- Doublets, clumps and dead cells can generate misleading data
- Method will be cell type or tissue dependent
- Practice makes perfect!
Fixation or Not
- Fixation holds cells in stasis but is this what you want?
- It will depend on:
- Local rules (infection)
- Time available (storage)
- Probe/fluorochrome to be used (Beware tandems!)
- Target of interest (surface vs. internal)
- Background autofluorescence
- Local rules (infection)
- Time available (storage)
- Probe/fluorochrome to be used (Beware tandems!)
- Target of interest (surface vs. internal)
- Background autofluorescence
Fixatives
- Alcohol
- Generally ethanol or methanol
- Dehydrating fixative
- Coagulates proteins
- Excellent for DNA
- Variable with antigens
- Aldehyde
- Generally formaldehyde
- Cross-linking fixative
- Good for antigens, GFP and fluorochromes
- Poor for DNA
Permeabilization
Where and Why?
- DNA
- Cytokines
- Intracellular proteins e.g. phospho-specific antibody
- Signaling pathways
- Cyclins
| Harsh | Gentle |
|---|---|
| Triton-X100 | Saponin |
| NP40 (IGEPAL) | Lysolecithin |
| Good for nuclear antigens | Good for cytoplasmic antigens |
Viability
- Dead cells in your sample can cause problems
- Cells with compromised membranes and loss of function will allow entry and binding of dye
- These dyes are either fluorescent DNA binding dyes or dyes that bind cellular proteins (amines)
- Can be used on unfixed or fixed cells
Doublet Discrimination
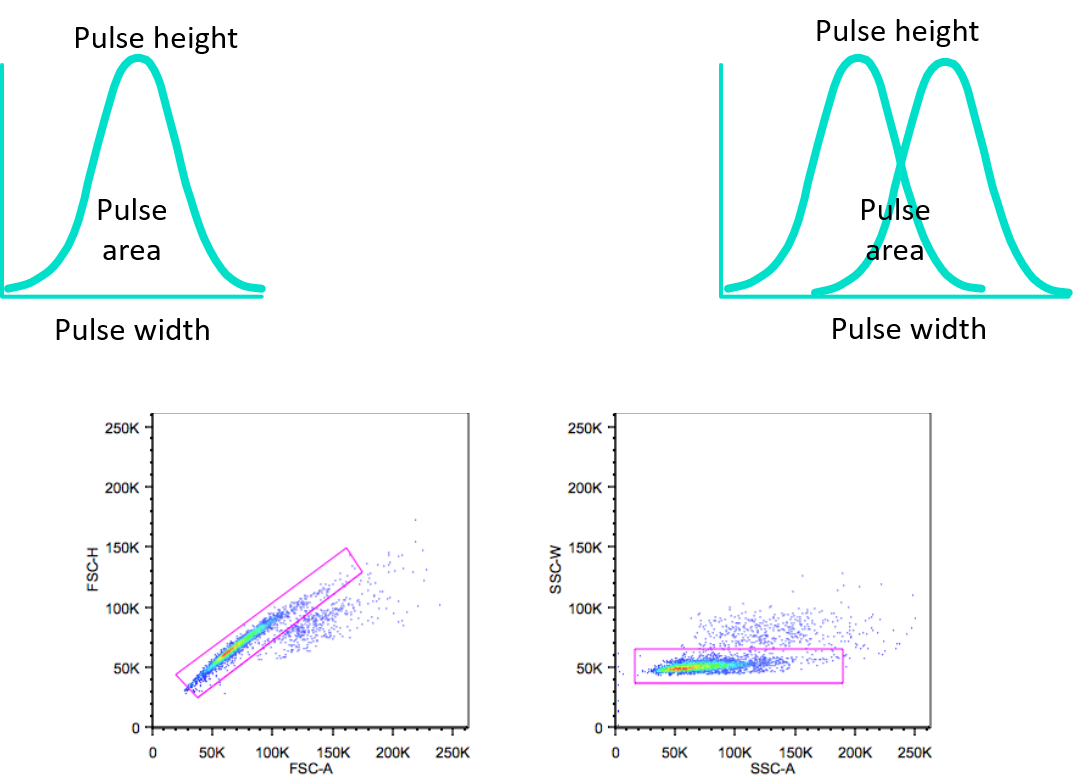
A note on Titrating your reagents
Why titrate?
- The best sample preparation can be ruined by poor sample staining
- You should titrate all of your antibodies and probes!
- Optimize your assay
- Enhance your data
- Improve consistency
- Save Money
- Optimize your assay
- Enhance your data
- Improve consistency
- Save Money
A note on Titrating your reagents
- Samples for titration should be identical to experimental samples
- Time - typically 20-30mins
- Temperature - any temperature from 4-37℃ can be used
- Staining volume - especially important if using relative concentration (e.g. 1:100)
- Try 4-6 doubling dilutions (e.g. 1:50 - 1:1600
- Quantitation with stain index (MFIpositive - MFInegative/ 2σnegative)
A word on controls
- Negative cells – to assess autofluorescence
- Single colour controls – to set compensation
- Isotype controls – not recommended
- Fluorescence Minus One controls (FMO) – gating control
Data Analysis
Several general programs exist:
- FlowJo (BectonDickinson)
- FCS Express (De Novo software)
- VenturiOne (Applied Cytometry)
- Kaluza (Beckman Coulter)
- Cytobank (look for cytobank community for a free but limited option)
Data Analysis: The Normal Distribution
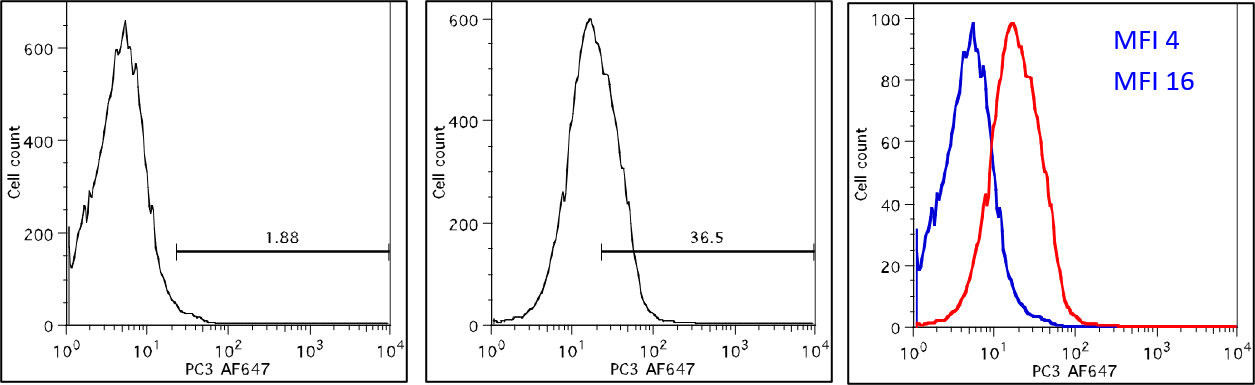
Data analysis: Plots and Numbers
- You are in control of your data
- Plots are just a VISUALISATION of the data
- We have to determine which populations in the data file are of interest
- We need to derive metrics to be able to compare samples
- Flow cytometry analysis is concerned with DESCRIPTIVE
statistics
- Enumeration of a subset - How MANY
- Measuring the level of fluorescence – How MUCH
Data Analysis: Percentage Positive
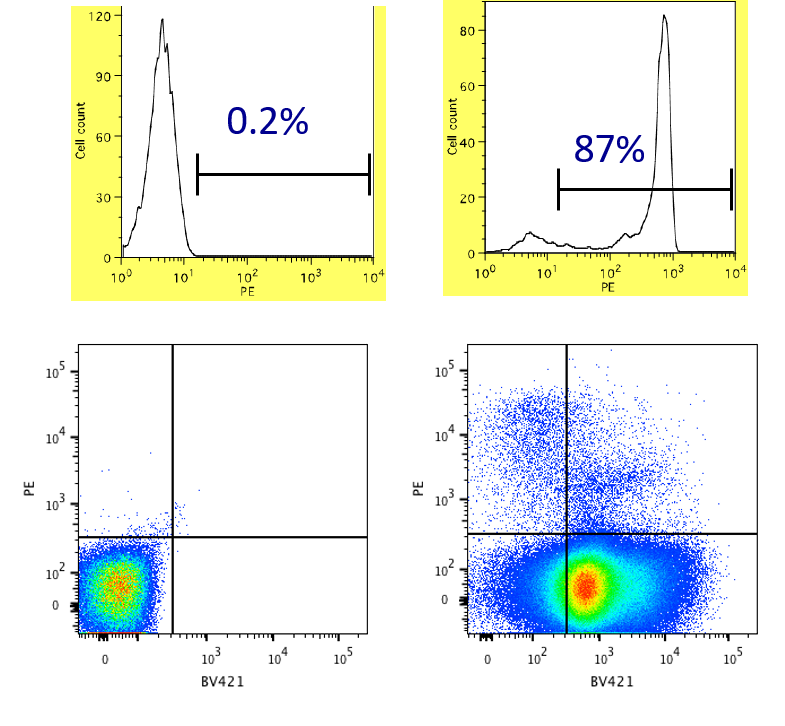
Summary
Flow cytometry is an extremely powerful technique BUT with some pitfalls for the unwary!
There's a lot to think about:
- The cytometer
- Fluorochromes used
- The sample preparation and conditions
- How to analyse the data